The semiconductor is divided into two types. One is Intrinsic Semiconductor and other is an Extrinsic semiconductor. The pure form of the semiconductor is known as the intrinsic semiconductor and the semiconductor in which intentionally impurities is added for making it conductive is known as the extrinsic semiconductor.
The conductivity of the intrinsic semiconductor becomes zero at room temperature while the extrinsic semiconductor is very less conductive at room temperature.
The detailed explanation of the two types of the semiconductor is given below.
Intrinsic Semiconductor
An extremely pure semiconductor is called Intrinsic Semiconductor. On the basis of the energy band phenomenon, an intrinsic semiconductor at absolute zero temperature is shown below:
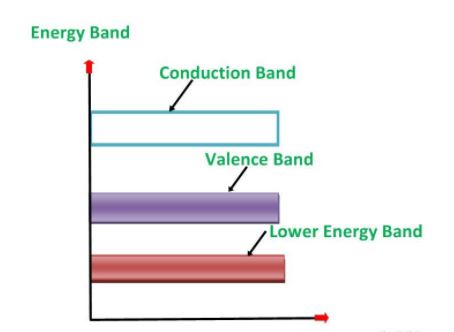
Its valence band is completely filled and the conduction band is completely empty. When the temperature is raised and some heat energy is supplied to it, some of the valence electrons are lifted to conduction band leaving behind holes in the valence band as shown below:
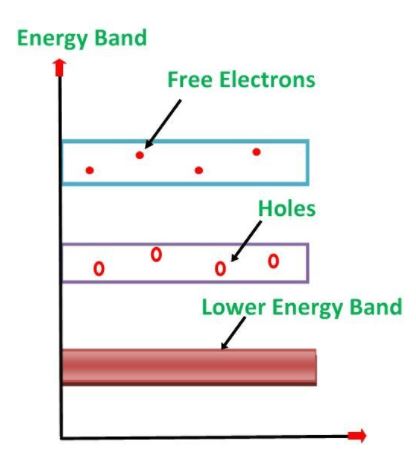
The electrons reaching at the conduction band move randomly. The holes created in the crystal also free to move anywhere. This behaviour of the semiconductor shows that they have a negative temperature coefficient of resistance.
This means that with the increase in temperature, the resistivity of the material decreases and the conductivity increases.
Extrinsic Semiconductor
A semiconductor to which an impurity at a controlled rate is added to make it conductive is known as an extrinsic semiconductor.
An intrinsic semiconductor is capable to conduct a little current even at room temperature, but it is not useful for the preparation of various electronic devices. Thus, to make it conducive a small amount of suitable impurity is added to the material.
Doping
The process by which an impurity is added to a semiconductor is known as Doping. The amount and type of impurity which is to be added to the material have to be closely controlled during the preparation of extrinsic semiconductor.
Generally, one impurity atom is added to 108 atoms of a semiconductor.
The purpose of adding impurity in the semiconductor crystal is to increase the number of free electrons or holes to make it conductive. If a Pentavalent impurity, having five valence electrons is added to a pure semiconductor a large number of free electrons will exist.
If a trivalent impurity having three valence electrons is added, a large number of holes will exist in the semiconductor.
Depending upon the type of impurity added the extrinsic semiconductor may be classified as n type semiconductor and p type semiconductor.